Our goal at EuroCan is to be a leader in the production of high quality, legally approved cannabis products and extracts for medical use in a range of formulations.

STATE OF THE ART FACILITY
MAÇÃO, PORTUGAL
- 31 Hectare Site with 3,600 mÇ Pharmaceutical Facility
- In-house QC Testing Lab, R&D Area
- Situated close to two large universities
- Latest extraction distillation, and purification technology installed
- Production from pilot scale to commercial scale batches
- Digital Facility
- Expandable footprint for multiple new products
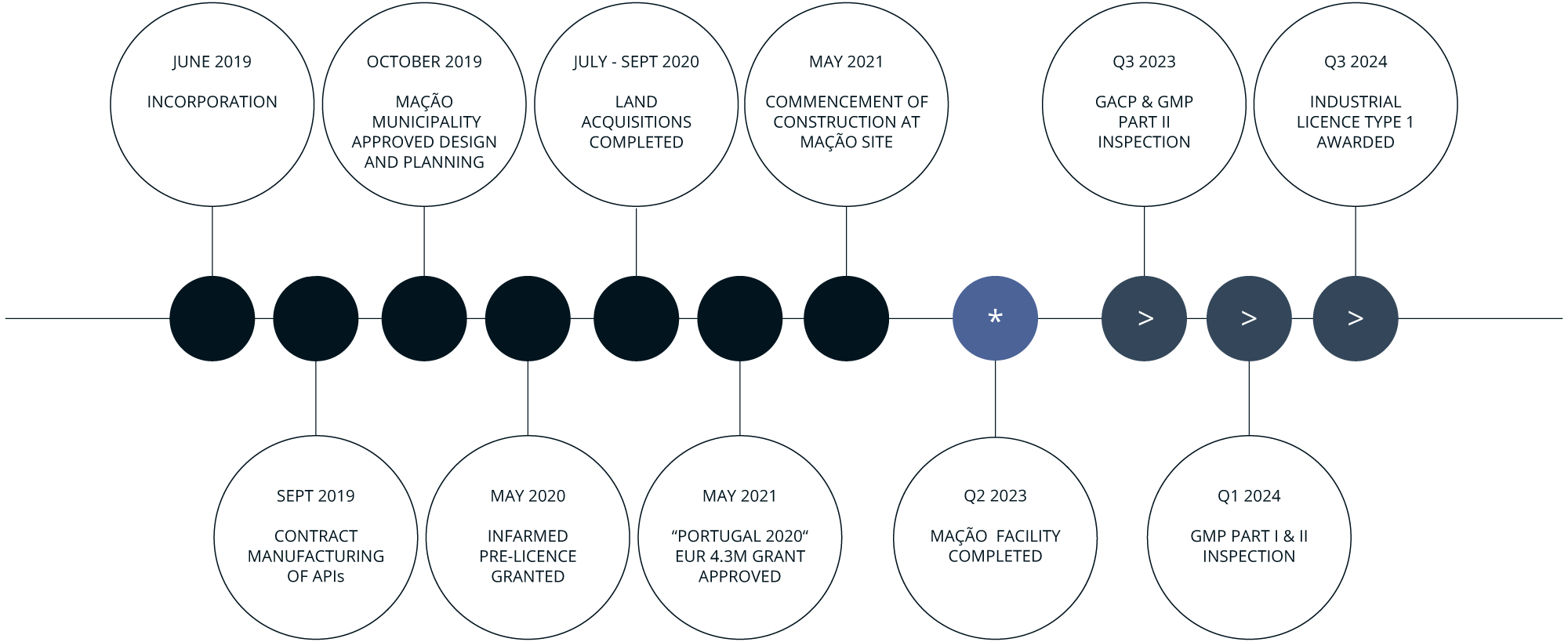
ACHIEVEMENTS AND MILESTONES
EXPANSION OPPORTUNITIES
ONCE GMP LICENSE HAS BEEN OBTAINED, EUROCAN WILL USE CAPACITY AT ITS FACILITY TO OFFER CONTRACT DEVELOPMENT AND MANFUACTURING (CDMO) SERVICES TO 3RD PARTIES ACROSS THE PHARMACEUTICAL VALUE CHAIN.
- Current site under-utilised
- Rezoning would allow up to 5 further facilities
- Large EU incentives available for right shoring of API manufacture
|
Phase 1 |
Cannabis Focussed |
|
Phase 2 |
CDMO Expansion |
|
Phase 3 |
Pharma Hub |


FACILITY AND CURRENT STATUS
- GACP Licenced Facility (2022)
- 2,300 m2 greenhouses construction after expansion
- Achieved first sales end of 2022
- Proprietary strain development
- Vertically Integrated
- Hydroponic & Bioponic Growing Methodology
- Continued revenue generation in 2023